An idea with its origins in ballistic prey catching—the way toads and chameleons snatch food with their tongues—may change fundamental views of muscle movement while powering a new approach to prosthetics.
After a decade of work, lead author Kiisa Nishikawa, Regents’ professor of biology at Northern Arizona University, and an international team of collaborators have published their hypothesis about spring-loaded muscles. Their paper, “Is Titin a ‘Winding Filament’? A New Twist on Muscle Contraction,” appears online in Proceedings of the Royal Society B.
The implications run deep in the world of movement neuroscience and hold promise for a burgeoning field of pioneers in bionics, exoskeleton robotic suits and prosthetics so advanced that the word “transhumanism” is becoming common.
“It turns out that our bodies are interacting with the environment all the time and our muscles can manage that interaction in a smart way without any intervention from the brain,” Nishikawa said. “There’s never been a model that explained how muscles are able to do that until now.”
Nishikawa’s approach lends credence to one of the two schools of thought that attempts to explain how the brain controls movement; namely, that the body and muscles “have a lot of passive-dynamic properties,” Nishikawa said, “and the job of the brain is to learn not to interfere with the passive dynamics.”
To demonstrate the concept, Nishikawa and her team built a physical model over the summer that “captures all the non-linear properties of muscle” that allow it to adapt instantaneously to changes in the environment, she said. Nishikawa is working with NAU mechanical engineering associate professor John Tester to design a working prototype.
The practical applications extend to “any device worn by humans where ultimate control comes from the human brain,” Nishikawa said. “The potential to improve those devices using this idea is good because the human brain expects its actuators to be muscles, so if you make them work like muscles, it’s bound to be more comfortable, efficient and stable.”
Getting to the spring-property conclusion took 10 years of grant-funded research and a complete retooling of Nishikawa’s lab, which had previously focused on the study of feeding in toads and chameleons to understand the power amplification in muscles that produces ballistic movements.
“We started asking the question, ‘What do muscles contribute to control?’” Nishikawa said. “Because of the emphasis on fast movement, we were really interested in looking at and accounting for the spring-like properties of muscles.”
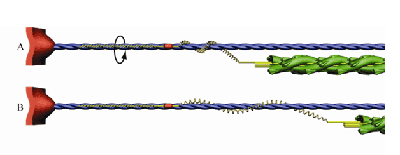
Nishikawa explained that most muscle theory was developed from 1925-1975, based on the idea that muscles stretch tendons, and the recovery of energy from the tendons led to high-power movement. But in the 1980s, the relatively late discovery of titin, the largest protein ever found, raised questions about that conventional view. A few other researchers proposed early ideas about titin as a spring, but their perspective did not include Nishikawa’s findings about titin winding.
“Our arms and legs are chock-full of tendons,” Nishikawa said, “but our heads and our jaws are not—there are some, but they are small and short. So we thought there had to be a spring inside the actual working unit of muscle.”
Titin, goes the hypothesis, is that spring. At a micron in length and 2 nanometers in diameter, it cannot be directly observed. “But we can model it,” Nishikawa said, “and it accounts for a lot of unexplained facts.” The computer modeling was performed by Nishikawa’s collaborators at the University of British Columbia.
Nishikawa pointed to recent prosthetics advances by iWalk and SpringActive as opportunities to put her ideas into action. “I would love to partner with companies like these to provide actuation that’s more compatible with how the human brain interacts with muscles,” she said. “Having been a scientist who’s done a lot of basic research, I’m excited that something practical might come of it.”