Mount Vesuvius. Krakatoa. Mount St. Helens.
Since the formation of Earth approximately 4.5 billion years ago, the convection and release of hot magma in its mantle layer has famously led to some of the deadliest eruptions in history. But what if the same explosions occurred when a geological feature and nearby chemical compounds became too cold?
Confirming this shocking phenomenon was the centerpiece of fifth-year doctoral student Shaelyn Raposa and recent NAU alumna Anna Engle’s study published last month in the Journal of Geophysical Research: Planets. Focusing on materials found on the icy glaciers of Pluto and the lakes of Titan, Saturn’s largest moon, the researchers initially set out to examine how these compounds, which exist as gases on Earth, behave in below-freezing temperatures.
Nantes/University of Arizona.
They were met with a surprise, however, when the chilly conditions of Wettaw’s Astrophysical Materials Laboratory led to drastic pressure spikes and minor explosions when their chemical mixtures began to freeze, an event the researchers dubbed an “outburst.”
“These outbursts occur when cooling a mixture, similar to the aftermath of leaving a soda in the freezer for too long,” Raposa said. “However, when it comes to geologic eruptive processes, we think of them as a consequence of warming something up, like volcanoes for example. In both cases, a change in temperature causes a change in pressure, which makes sense, but we weren’t expecting to see a pressure spike after our mixtures froze.”
Completing the study in 2023, Raposa and Engle made mixtures with varying levels of nitrogen, ethane, carbon monoxide or methane—all compounds found in liquid form on Pluto and Titan—and injected them into aluminum alloy sample cells. These cells were then cooled in a helium refrigerator and temperature-controlled using two nichrome heaters, replicating the minus-300-degree temperatures of the outer solar system.
Using spectroscopy, the researchers could then confirm the exact conditions that led to phase changes in each compound mixture, from gaseous forms to solid ices. This study was a rare effort to accurately replicate extraterrestrial conditions on Earth, only made possible by a unique lab space at NAU.
“It’s expensive to send physical missions to places like Pluto and Titan to study the surface, which is why it’s nice to be able to study how these materials behave in a laboratory setting,”Raposa said. “There are difficulties, however, because you need a lab setup similar to ours designed to study mixtures at low temperatures. Not many of these exist, and the result is that there has been very little prior research studying the behaviors of these materials.”
When the mixtures were at or below their freezing points, the fluid trapped below the solidifying ice began bursting and bulging, representing a release of latent heat and a pressure spike that outmatched the strength of the confining solid.
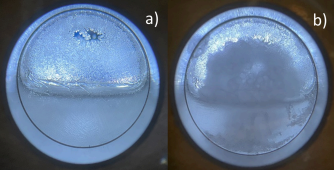
This reaction could possibly be the answer to countless explosive mysteries plaguing our solar system and beyond, from ice or mud volcanism on Mars to sharp-edge depressions on Titan’s surface.
“Under the right conditions, we might see eruptive phenomena caused by cooling on both icy and rocky worlds,” Raposa said. “Outbursts like the ones we saw in the lab may provide alternative explanations for the explosion craters found in Siberia and perhaps the geysers and plumes detected on Europa, a moon of Jupiter, and Enceladus, another moon of Saturn.”
Although magma and grueling heat may not be prominent factors on other planetary bodies billions of miles away, Raposa and Engle’s study ensures scientists won’t have to put their explorations on ice.
Data regarding how temperature and pressure changes influence compounds outside of Earth can prove critical for making accurate models of places like Pluto and Titan, ultimately leading to more discoveries surrounding each astronomical object.
With this study’s valuable insights into thermodynamics, astronomers are one step closer to bringing the solar system’s cosmic rumbling giants to our terrestrial laboratories.
Top photo credit: NASA/Johns Hopkins University Applied Physics Laboratory/Southwest Research Institute



