In Tim Becker’s lab, a “patient” lies on the surgical table with a blood clot lurking in a brain vessel. It’s a scary scenario, akin to a stroke, one of the United States’ top causes of death and disability.
But here’s the twist: the patient isn’t a real person, just a collection of tubes and pumps circulating fluids. And Becker isn’t a surgeon—he’s a mechanical engineer. With his team, Becker aims to develop and test medical devices that can better treat stroke patients in the critical hours following a stroke.
“Stroke’s a pretty big research area, and current treatments are not that great,” said Becker, who leads the Bioengineering Devices Lab at NAU. “The devices coming out now are evolving really quickly, and we’re on the ground floor of that.”
He’s been working in this space, both in the lab and in industry, since the start of his career. Now in addition to innovation, Becker is training the next generation of medical device developers. A dozen graduate and undergraduate students in bioengineering, biology, physical therapy, chemistry and materials science collaborate on developing devices and the systems needed to test them. They graduate with hundreds of hours of hands-on experience, collaboration with industry, co-authored journal articles, patents, federal funding and job offers
It’s an exciting place to be.
The road to 100% success rate in stroke care
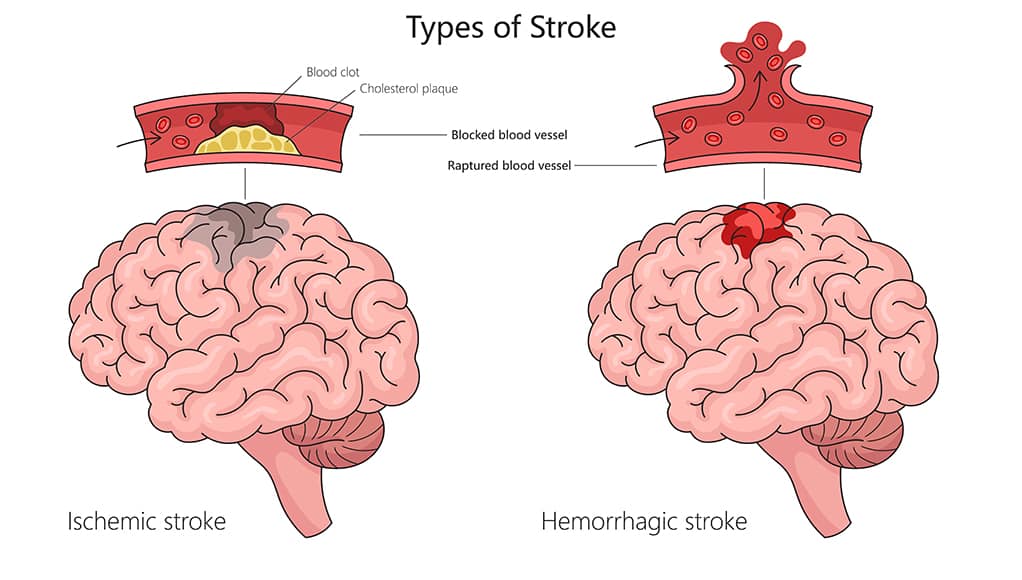
Becker’s lab is working on medical devices for both ischemic and hemorrhagic strokes. Ischemic strokes, which are caused by a blood clot in the brain, are treated either by injecting a clot-busting drug into the patient or inserting a catheter that will allow doctors to vacuum out the clot. The suction process, developed about a decade ago, has 50-60% effectiveness—much higher than the drug, but not high enough.
Becker and his students are developing suction devices that can grab the entire clot. Becker compared this procedure to trying to suck a muffin top into a tube—with enough suction, some of the muffin will get into the tube, and it’s possible that all of it can be sucked in. But luck plays a big role: Often, some of it is missed.
“We’re working on a catheter with a tip that can adapt to the shape of the vessel and grab all of the clot, not just some of it,” Becker said. “Ours would open up and grab the entire clot and suck it in on the first pass instead of letting pieces go downstream and potentially cause another stroke. This could really increase effective treatment.”
A hemorrhagic stroke occurs when an aneurysm in the brain bursts. Right now, a patient has a 15% chance of survival after a hemorrhagic stroke. Aneurysms, when they are caught before bursting, can be treated surgically; a coil is placed at the mouth of the aneurysm, which reinforces the weakened vessel. A balloon is inserted into the vessel to hold the coil in place (using a glue-like material also developed in Becker’s lab). The issue? The blood vessel is blocked for about 10 minutes, keeping blood from getting to the brain.
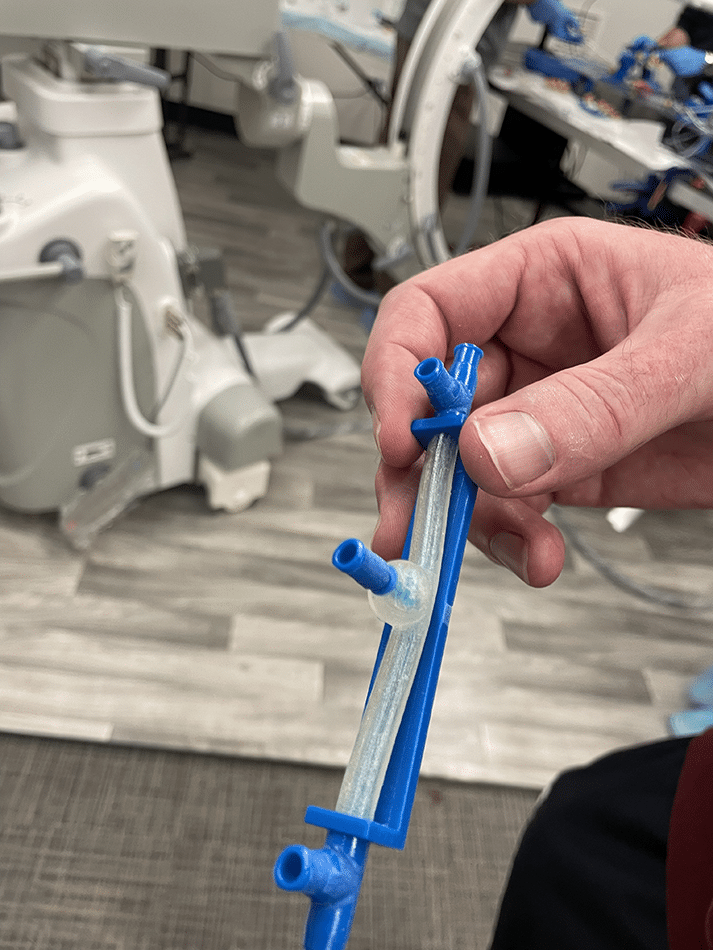
Becker’s lab is developing a novel balloon mesh, which would function the same way as a balloon but be porous, allowing blood to flow through the vessels normally and causing the vessel to heal over the entrance to the aneurysm. The team has built three prototypes of this material, and they plan to create two more.
Testing on simulated patients
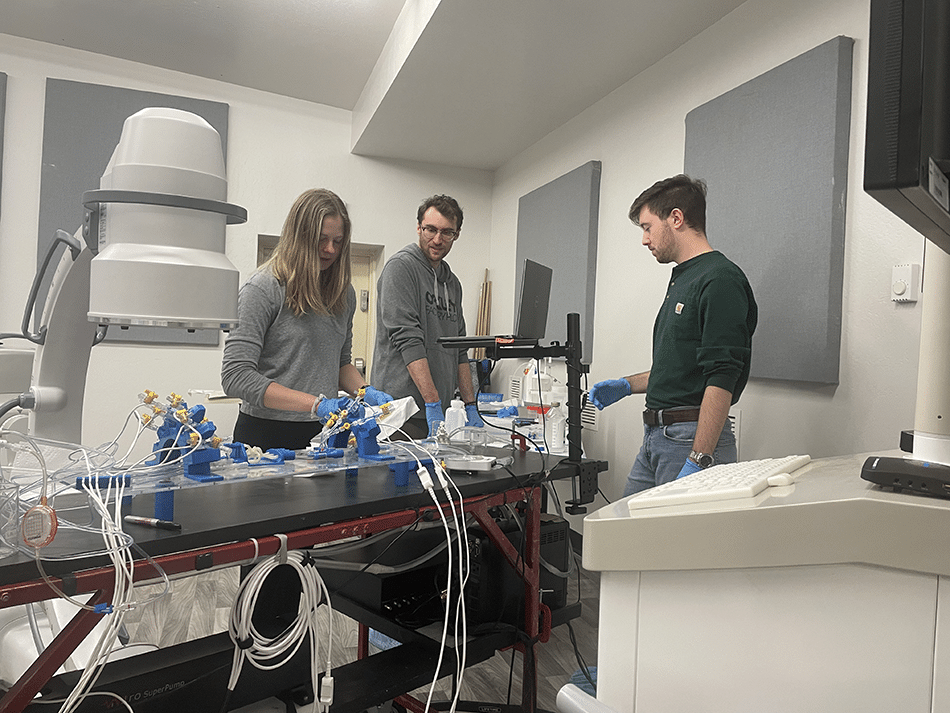
The “patient” in Becker’s lab has been carefully created to mimic a human. The “blood vessels” are 3D-printed with a flexible material that reacts to blood flow as human vessels do. The “blood” is a liquid with a similar mechanical makeup as human blood, which is moved through the vessels by a pump system that can be programmed to mimic the heart of a child or adult, a man or a woman of different ages. (They can’t program it to different ethnicities—yet.) It’s all hooked up to a computer that measures pulse, blood pressure, blood flow and more.
The biohazard-free setup allows Becker and his team to get in there with their prototypes, testing what works, what needs to be improved, switching variables—good old trial and error.
In fact, in February, Becker’s team conducted a four-hour video call with Harvard Medical School researchers, testing various devices Harvard had sent. The doctors had found that when they put the devices into live patients, the patients weren’t responding as the doctors anticipated. They wanted to know how to get those outcomes, so Becker pulled up the camera to his simulated patient and they worked through the devices, watching measurements on the computer screen.
ATTACing racial and gender disparities in health outcomes
For the last 50 years, medical devices have been made for the average white man. Turns out, that isn’t working—not only because of the biological differences among genders and races, but also because the “average white man” is a medical myth. Every human body is different, and to be effective, medical devices need to be adaptable to different body types.
Holly Berns, a Ph.D. student in bioengineering, is developing a prototype, called the ATTAC catheter, that is more adaptive—instead of one-size-fits-all, it’ll be one device that can be adjusted by size. Having a single device that can treat many body types is a cost-saving measure for hospitals that also will lead to better outcomes for stroke patients: Right now, men have about a 60% survival rate, while women have a survival rate of about 30-45%.
Berns, who received $500,000 in funding from the National Institutes of Health, graduates in about a year, and her hope is that she’ll be able to take the ATTAC catheter all the way through development, testing and the patent process and into production on the commercial side.
“I joined this lab four years ago, and right after that, my partner at the time had a stroke,” she said. “Sitting in the hospital room with him, knowing this background on stroke, it is unacceptable that we’re only at 60%. We can do better—we need to do better.”
Heidi Toth | NAU Communications
(928) 523-8737 | heidi.toth@nau.edu




